Published online Aug 21, 2013. doi: 10.3748/wjg.v19.i31.5159
Revised: July 4, 2013
Accepted: July 12, 2013
Published online: August 21, 2013
AIM: To investigate the effects of suberoylanilide hydroxamic acid (SAHA) on proliferation and apoptosis of a human hepatocellular carcinoma cell line (HepG2.2.15) and hepatitis B virus (HBV) replication.
METHODS: HepG2.2.15 cells were treated with different concentrations of SAHA. Cell morphology was examined by confocal laser scanning microscopy, and cell proliferation was determined using a MTT colorimetric assay. Flow cytometry was used to detect apoptosis and determine cell cycle phase, while hepatitis B surface antigen and hepatitis B e antigen content were measured using chemiluminescence. Reverse transcription polymerase chain reaction was performed to measure HBV DNA in cell lysate.
RESULTS: Cell proliferation rates were significantly reduced by the addition of SAHA. The inhibitory effect of SAHA on cell proliferation was both time- and dose-dependent. After 24 h of treatment with SAHA, the early cell apoptotic rate increased from 3.25% to 21.02% (P = 0.041). The proportion of G0/G1 phase cells increased from 50.3% to 65.3% (P = 0.039), while that of S phase cells decreased from 34.9% to 20.6% (P = 0.049). After 48 h of treatment, hepatitis B surface antigen and hepatitis B e antigen content increased from 12.33 ± 0.62 to 25.42 ± 2.67 (P = 0.020) and 28.92 ± 1.24 to 50.48 ± 1.85 (P = 0.026), respectively. Furthermore, HBV DNA content increased from 4.54 ± 0.46 to 8.34 ± 0.59 (P = 0.029).
CONCLUSION: SAHA inhibits HepG2.2.15 cell proliferation, promotes apoptosis, and stimulates HBV replication. In combination with anti-HBV drugs, SAHA may potentially be used cautiously for treatment of hepatocellular carcinoma.
Core tip: HepG2.2.15 cells were treated with different concentrations of suberoylanilide hydroxamic acid (SAHA). Hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg) content were measured using chemiluminescence. Reverse transcription polymerase chain reaction was performed to measure hepatitis B virus (HBV) DNA in cell lysate. Results found that, the inhibitory effect of SAHA on cell proliferation was both time- and dose-dependent. After 24 h of treatment, the early cell apoptotic rate increased. After 48 h of treatment, HBsAg and HBeAg content both increased. Furthermore, HBV DNA content increased. In combination with anti-HBV drugs, SAHA may potentially be used cautiously for treatment of hepatocellular carcinoma.
- Citation: Wang YC, Yang X, Xing LH, Kong WZ. Effects of SAHA on proliferation and apoptosis of hepatocellular carcinoma cells and hepatitis B virus replication. World J Gastroenterol 2013; 19(31): 5159-5164
- URL: https://www.wjgnet.com/1007-9327/full/v19/i31/5159.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i31.5159
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors. The worldwide incidence of HCC ranks fifth out of all malignant tumors, and the number of patients with HCC in China accounts for more than half of total cases in the world[1]. Etiological factors of HCC vary for different countries and areas. Histone deacetylase inhibitors (HDACIs) are a series of new anticancer drugs with a wide scope of application. In recent years, HDACIs have generated considerable interest due to their high efficiency to inhibit a variety of solid tumors with low toxicity[2-6]. In the current study, the effects of suberoylanilide hydroxamic acid (SAHA), a potent HDACI, on proliferation and apoptosis of human HCC cells HepG2.2.15 and hepatitis B virus (HBV) replication were investigated. The study objective was to characterize a potentially new treatment option for HCC.
HepG2.2.15 cells (obtained from the Cell Center of the Chinese Academy of Medical Sciences; prepared by transfection of HepG2 cells with HBV genome) were maintained in DMEM (HyClone Laboratories, Inc., New England, United States) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 U/mL streptomycin and 380 mg/L G418 in a thermostatic and sealed incubator (37 °C, 5% CO2). About 100 mmol/L SAHA (Sigma-Aldrich Corp, Missouri, United States) in dimethylsulfoxide (DMSO) was prepared and stored at -20 °C until further use. HepG2.2.15 cells were divided into a control group and several treatment groups to receive different concentrations of SAHA. The adherent cells were washed down with 0.25% trypsin, followed by passage.
An MTT colorimetric assay was used to monitor inhibition of cell proliferation by the addition of different concentrations of SAHA to cell culture medium. For three 96-well plates, 100 μL HepG2.2.15 cells (1 × 105 cells/mL) was added to each well and incubated for 12 h at 37 °C in 5% CO2. Once cells were adhered to the wells, SAHA was added to a final concentration of 2.5, 5, 7.5 or 10 μmol/L. Wells without SAHA were used as negative controls. After the addition of SAHA, a culture plate was incubated for 24, 48 or 72 h. Cell morphology was examined by confocal laser scanning microscopy (CLSM). Then, 20 μL of MTT (5 mg/mL) was added to each well. After incubation for 4 h, 150 μL of DMSO was added, followed by mixing for 10 min. Lastly, absorbance (A) at 490 nm was measured using a microplate reader. The inhibition rate of cell proliferation was calculated as follows: Cell proliferation inhibition rate (%) = (1 - ASAHA group/ANegative control group) × 100%.
Control group and SAHA groups (2.5 and 5 μmol/L) were cultured for 24 and 48 h respectively. The single cell suspension was then prepared. After centrifugation at 2000 g for 5 min, the cell pellet was resuspended in 0.5 mL PBS (final concentration, 1-5 × 105 cells/mL). For detection of apoptosis, binding buffer (500 μL) and 5 μL annexin V-fluorescein isothiocyanate (Annexin V-FITC) were added to the cell resuspension, followed by 5 μL propidium iodide (PI) (Nanjing KGI Biological Technology Co., Ltd., Nanjing, China). After incubation for 5-15 min (room temperature, avoiding light), samples were subjected to flow cytometry (FCM). For determination of cell cycle phase, 5 mL of obtained cell resuspension was infused into 70% cold ethanol, followed by fixation at 4 °C overnight. During the next day, the cell solution was centrifuged at 800 r/min for 15 min, followed by two phosphate buffer saline (PBS) washes and resuspension in 0.4 mL PBS. RNaseA was added to a final concentration of 50 μg/mL, followed by digestion for 30 min in a 37 °C water bath. Lastly, PI was added to a final concentration of 65 μg/mL, followed by incubation for 30 min. After filtration through a nylon mesh, FCM was conducted.
HepG2.2.15 cells (2.5 × 105 cells/mL) were plated onto a 6-well plate. In triplicate, 1 μL SAHA (7.5 μmol/L) or an equivalent volume of DMSO was added to an individual well. After incubation for 72 h, cells were centrifuged at 800 r/min for 5 min. The supernatant was collected, and the hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg) content were quantitated using quantitative chemiluminescence detection kits in i4000sR automatic chemiluminescence immunoassay analyser (R.D. Abbott Company, Inc., California, United States).
A 6-well cell culture plate was prepared as previously described above. HBV negative and positive controls were prepared as follows: 100 μL of cell supernatant was mixed with an equal amount of DNA extraction liquid (shaking for 15 s), followed by centrifugation at 12000 g for 10 min to remove supernatant. Then, 20 μL of DNA extraction liquid was added to the sediment, followed by incubation for 10 min in a 100 °C water bath.
HBV-polymerase chain reaction (PCR) reaction liquid (35.6 μL) and Taq enzyme (0.4 μL) were mixed in a 0.2 mL Eppendorf tube. Two μL of treated sample supernatant was then added to each tube and centrifuged at 8000 g for several seconds. Quantitative fluorescent PCR was performed under the following conditions: 95 °C for 3 min; 94 °C for 15 s (40 cycles); 60 °C for 30 s (40 cycles).
Data were expressed as mean ± SD. Statistical analysis was performed using SPSS 16.0 statistical software. Single factor analysis of variance and t tests were conducted for comparison among multiple groups. P < 0.05 was considered as statistically significant.
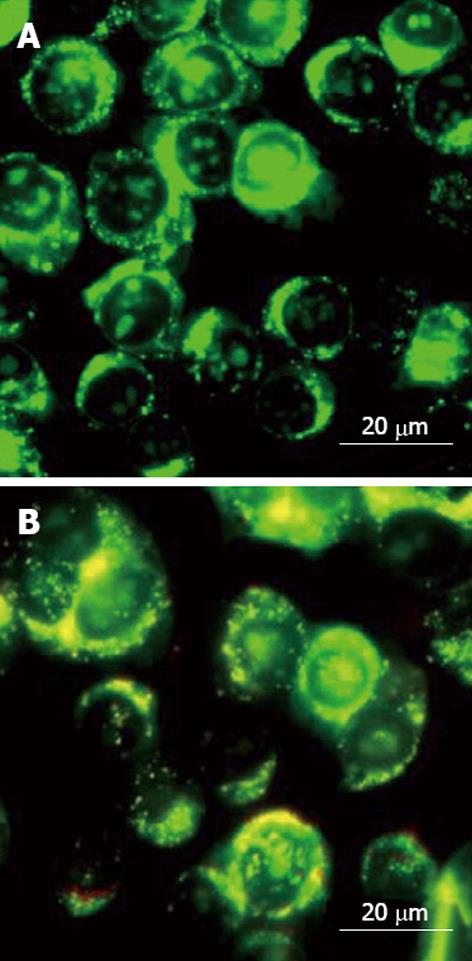
CLSM showed that proliferation of untreated HepG2.2.15 cells was rapid, and the cells were compactly arranged. The adjacent cells fused into pieces, with clear edge. The cytoplasm was small, with a large nucleus. In SAHA-treated groups, cell proliferation rate was significantly slower. There were sparse adherent cells, with blurred configuration. The cytoplasm increased, presenting morphological changes similar to normal cells (Figure 1).
Multiple concentrations of SAHA could inhibit proliferation of HepG2.2.15 cells, and the inhibitory rate increased with increasing concentrations of SAHA (P < 0.05). With each SAHA concentration, the inhibition rate gradually increased with prolonged treatment time (P < 0.05). Taken together, the inhibitory effect of SAHA on cell proliferation was time- and dose-dependent (Table 1).
| SAHA (μmol/L) | 24 h | 48 h | 72 h |
| 2.5 | 8% ± 0.54% | 15% ± 1.52% | 23% ± 1.39% |
| 5.0 | 13% ± 0.63% | 22% ± 1.68% | 34% ± 1.61% |
| 7.5 | 28% ± 1.56% | 39% ± 1.67% | 50% ± 1.70% |
| 10.0 | 42% ± 1.72% | 51% ± 1.82% | 66% ± 1.76% |
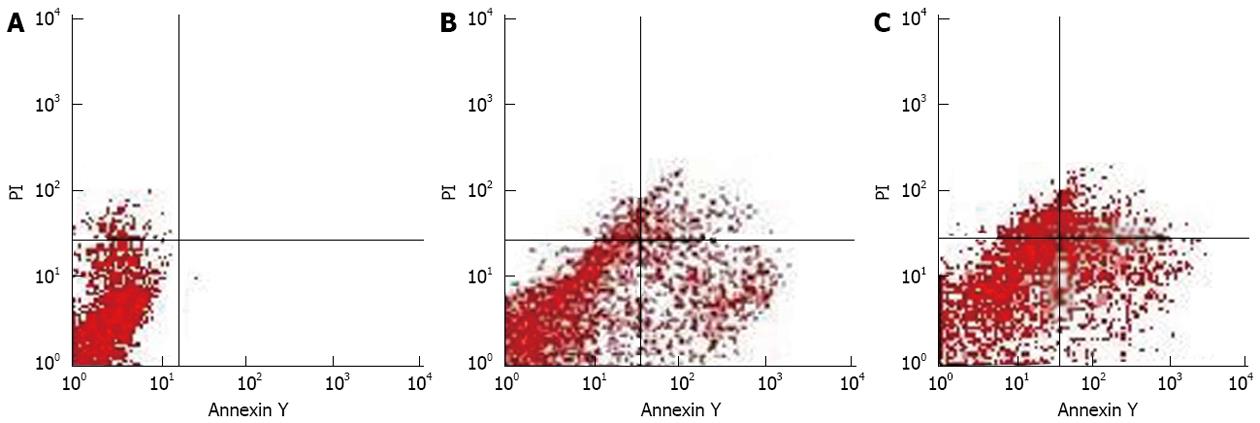
After 24 h of treatment with 2.5 μmol/L SAHA, early apoptosis rate of HepG2.2.15 cells increased from 3.25% to 16.28% (P = 0.032), and the middle-late apoptosis rate increased from 1.08% to 5.16% (P = 0.035). In the 5 μmol/L SAHA group, early and middle-late apoptosis rate increased from 3.25% to 21.02% (P = 0.041) and 1.08% to 10.70% (P = 0.045), respectively (Table 2 and Figure 2). After 24 h of treatment with 2.5 and 5 μmoL/L SAHA, the proportion of G0/G1 phase cells increased from 50.3% to 69.9% and 65.3%, respectively, and the proportion of S phase cells decreased from 34.9% to 22.3% and 20.6%, respectively. Most cells were arrested in the G0/G1 phase (Table 3).
| Group | 24 h | 48 h | ||
| Early apoptosis rate | Middle-late apoptosis rate | Early apoptosis rate | Middle-late apoptosis rate | |
| Control | 3.25% | 1.08% | 3.58% | 1.26% |
| 2.5 μmol/L SAHA | 16.28% | 5.16% | 23.06% | 8.42% |
| 5.0 μmol/L SAHA | 21.02% | 10.70% | 26.44% | 17.55% |
| Group | 24 h | 48 h | ||||
| G0/G1 | S | G2/M | G0/G1 | S | G2/M | |
| Control | 50.3% | 34.9% | 14.8% | 46.3% | 38.2% | 15.5% |
| 2.5 μmol/L SAHA | 69.9% | 22.3% | 7.8% | 70.9% | 26.1% | 3.0% |
| 5.0 μmol/L SAHA | 65.3% | 20.6% | 14.1% | 68.9% | 25.8% | 5.3% |
Positive expression of HBsAg and HBeAg in the SAHA group and control group, respectively, was observed. After 48 h of treatment with SAHA, HBsAg and HBeAg content increased from 12.33 ± 0.62 to 25.42 ± 2.67 (P = 0.020) and 28.92 ± 1.24 to 50.48 ± 1.85 (P = 0.026), respectively, and HBV DNA content increased from 4.54 ± 0.46 to 8.34 ± 0.59 (P = 0.029).
Abnormality of any step of epigenetics can affect gene expression or function, leading to the occurrence of disease, such as cancer. As a main epigenetic pattern, histone acetylation is closely related with tumor occurrence. HDACIs are often used to alter histone acetylation for treatment of cancer[7-9]. SAHA is a broad-spectrum HDACI, and was approved for treating T-cell lymphoma in 2006 (in phase I and II clinical trial). It has obvious inhibitory effects on histone deacetylase, and can inhibit the growth of HCC cells by arresting cell cycle progression and inducing cell differentiation and apoptosis. HDACIs have been shown to exhibit a broad-spectrum inhibitory activity on blood and solid tumors[10,11].
Unrestricted division and proliferation is an important feature of tumor cells. Detection of an inhibitory effect on tumor cell proliferation is a basic index for screening of anti-tumor drugs. In this study, CLSM and a MTT colorimetric assay were used to show that cell proliferation rates was significantly decreased by treatment with SAHA. Specifically, the number of cells was reduced, and adherent cells became sparse. Time- and dose-dependencies of SAHA inhibition on cell proliferation were evident. The cytoplasm increases, presenting morphological changes similar to normal cells, which is consistent with results from a previous study[12].
Apoptosis is programmed cell death. The process of apoptosis and the clearance of apoptotic cells is one of the most important factors for maintaining liver health. In this study, after 24 h of treatment with 5 μmol/L SAHA, early apoptosis rate and middle-late apoptosis rate of HepG2.2.15 cells increased from 3.25% to 21.02% (P = 0.029) and 1.08% to 10.70% (P = 0.045), respectively, indicating that SAHA may interfere with the balance between apoptosis and anti-apoptosis, induce the expressions of pro-apoptotic genes (Bmf, Bim, TRAIL and DR5)[13], and activate the expression of transcription factor E2F1. Furthermore, SAHA can induce the expression of apoptosis signal-regulating kinase 1 (ASK1), which promotes apoptosis of tumor cells through the death receptor and intracellular apoptotic pathways[14,15]. In addition, SAHA can activate the expression of pro-apoptotic proteins, including Bax and Bak, and inhibit expression of anti-apoptosis proteins, including Bcl-2 and Bcl-xL, thus inducing apoptosis of tumor cells[16]. In the extracellular apoptotic pathway, activated caspase-8 can cleave Bid to truncated Bid (tBid), as well as cause Cyt C release and Bax expression, leading to activation of caspase-9 and caspase-3. Caspase-3 can promote activation and cleavage of PARP to subsequently activate the intracellular apoptotic pathway[17]. SAHA has been shown to induce transcription of CDK inhibitor p21/wafl in T24 bladder cancer cells, reducing proliferation and increasing apoptosis[14].
Abnormality of cell cycle regulation is one of intrinsic factors for tumor occurrence. HDACIs can arrest tumor cell cycle, inhibiting growth. Results of this study show that, after 24 h of treatment with 5 μmoL/L SAHA, the proportion of G0/G1 phase cells increases from 50.3% to 65.3%, and the proportion of S phase cells decreases from 34.9% to 20.6%. Most cells were arrested in the G0/G1 phase and induced to undergo apoptosis. This may be related to increased expression of CDK inhibitor p21/waf1, which is induced by SAHA treatment. Nearly all HDACIs can induce expression of p21/waf1 to inhibit the activities of cyclin and CDK, resulting in cell cycle arrest and inhibition of differentiation. In addition, SAHA has been shown to influence expression of p27. After SAHA treatment, the degree of histone acetylation is elevated, stabilizing the activity of p53 (an important intracellular tumor suppressor protein) and leading to cell cycle arrest[18-20]. The Ras-Raf-MEK-ERK pathway is closely related with tumor cell proliferation. ERK can be activated by various growth factors, leading to interaction with transcription factors (mitogen, c-Jun, c-fos, c-Myc, cERK1) and nuclear proteins to promote the transcription and expression of a variety of oncogenes and genes related to cell cycle regulation, thus promoting cell proliferation and inhibiting apoptosis[21-23].
HBV is a risk factor for development of HCC. An epidemiological survey demonstrated that the carrying rate of HBsAg in China is 7.18%. HBV can be actively replicated in patients with HCC, causing further liver damage[24-27]. HepG2.2.15 cells can continuously excrete intact HBV Dane particles into culture media. Upon treatment with SAHA, HBsAg and HBeAg content were 2.06 and 1.75 times greater than the control group, respectively, and HBV DNA content was 1.83 times greater than the control group. Taken together, SAHA stimulated replication of HBV. Histone acetylation is involved in regulation of gene transcription. After treatment with SAHA, the level of histone acetylation in HBV DNA is increased, and chromosome structure became incompact. This facilitates the combination of transcription factor with HBV DNA polymerase, thus stimulating HBV replication. However, this mechanism needs further validation. SAHA is an effective drug for HBV-negative HCC patients, but should be cautiously used in HBV-positive HCC patients in combination with anti-HBV drugs.
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors in the world. Its occurrence is related to the multiple-step development process of different genetic alterations. At present, there is no effective treatment method. Suberoylanilide hydroxamic acid (SAHA) is a newly discovered anti-tumor drug which has broad application prospect. It exhibits inhibitory effect of tumor growth, which is been further confirmed in clinical trials.
Histone deacetylase inhibitors (HDACIs) are a class of new anticancer drugs emerging in recent years, which has attracted widespread attention. Previous clinical trials find that, SAHA has broad-spectrum anti-hematological and solid tumor activities, with good tolerance. However, the effect of SAHA on hepatitis B virus (HBV) replication has not been reported.
SAHA inhibits HepG2.2.15 cell proliferation, promotes apoptosis, and stimulates HBV replication. In combination with anti-HBV drugs, SAHA may potentially be used cautiously for treatment of hepatocellular carcinoma.
SAHA has been applied in previous clinical trials. Results show that, it has broad-spectrum anti-hematological and solid tumor activities. SAHA can inhibit HepG2.2.15 cell proliferation, deduce the differentiation, and promote the apoptosis. At the same time, it can stimulate the replication of HBV. Therefore, SAHA should be cautiously used for treatment of HCC, and be combined with anti-HBV drugs if necessary. It can be used in the treatment of HBV-negative HCC patients.
HCC is one of the most common malignant tumors, and HDACIs are a series of new anticancer drugs with a wide scope of application. In this manuscript, the authors investigated the effects of suberoylanilide hydroxamic acid, a potent HDACI on proliferation and apoptosis of a human hepatocellular carcinoma cell line (HepG2.2.15) and HBV replication. The manuscript is very well written.
P- Reviewers Balachandar V, Germani G S- Editor Wang JL L- Editor A E- Editor Li JY
| 1. | Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533-543. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Johnstone RW, Licht JD. Histone deacetylase inhibitors in cancer therapy: is transcription the primary target? Cancer Cell. 2003;4:13-18. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Kelly WK, O’Connor OA, Marks PA. Histone deacetylase inhibitors: from target to clinical trials. Expert Opin Investig Drugs. 2002;11:1695-1713. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Nishioka C, Ikezoe T, Yang J, Takeuchi S, Koeffler HP, Yokoyama A. MS-275, a novel histone deacetylase inhibitor with selectivity against HDAC1, induces degradation of FLT3 via inhibition of chaperone function of heat shock protein 90 in AML cells. Leuk Res. 2008;32:1382-1392. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Kang MR, Kang JS, Han SB, Kim JH, Kim DM, Lee K, Lee CW, Lee KH, Lee CH, Han G. A novel delta-lactam-based histone deacetylase inhibitor, KBH-A42, induces cell cycle arrest and apoptosis in colon cancer cells. Biochem Pharmacol. 2009;78:486-494. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Sun C, Zhou J. Trichostatin A improves insulin stimulated glucose utilization and insulin signaling transduction through the repression of HDAC2. Biochem Pharmacol. 2008;76:120-127. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Gahr S, Peter G, Wissniowski TT, Hahn EG, Herold C, Ocker M. The histone-deacetylase inhibitor MS-275 and the CDK-inhibitor CYC-202 promote anti-tumor effects in hepatoma cell lines. Oncol Rep. 2008;20:1249-1256. [PubMed] [Cited in This Article: ] |
| 8. | Habold C, Poehlmann A, Bajbouj K, Hartig R, Korkmaz KS, Roessner A, Schneider-Stock R. Trichostatin A causes p53 to switch oxidative-damaged colorectal cancer cells from cell cycle arrest into apoptosis. J Cell Mol Med. 2008;12:607-621. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene. 2007;26:5541-5552. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Duvic M, Talpur R, Ni X, Zhang C, Hazarika P, Kelly C, Chiao JH, Reilly JF, Ricker JL, Richon VM. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL). Blood. 2007;109:31-39. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Johnstone RW. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov. 2002;1:287-299. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Li QF, Ouyang GL, Liu QR, Hong SG. Tachyplesin-induced differentiation of human hepatocarcinoma cell line SMMC-7721. Aizheng. 2002;21:480-483. [PubMed] [Cited in This Article: ] |
| 13. | Kim HJ, Bae SC. Histone deacetylase inhibitors: molecular mechanisms of action and clinical trials as anti-cancer drugs. Am J Transl Res. 2011;3:166-179. [PubMed] [Cited in This Article: ] |
| 14. | Tan J, Zhuang L, Jiang X, Yang KK, Karuturi KM, Yu Q. Apoptosis signal-regulating kinase 1 is a direct target of E2F1 and contributes to histone deacetylase inhibitor-induced apoptosis through positive feedback regulation of E2F1 apoptotic activity. J Biol Chem. 2006;281:10508-10515. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Kim H, Kim SN, Park YS, Kim NH, Han JW, Lee HY, Kim YK. HDAC inhibitors downregulate MRP2 expression in multidrug resistant cancer cells: implication for chemosensitization. Int J Oncol. 2011;38:807-812. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Park KC, Kim SW, Park JH, Song EH, Yang JW, Chung HJ, Jung HJ, Suh JS, Kwon HJ, Choi SH. Potential anti-cancer activity of N-hydroxy-7-(2-naphthylthio) heptanomide (HNHA), a histone deacetylase inhibitor, against breast cancer both in vitro and in vivo. Cancer Sci. 2011;102:343-350. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Ellis L, Bots M, Lindemann RK, Bolden JE, Newbold A, Cluse LA, Scott CL, Strasser A, Atadja P, Lowe SW. The histone deacetylase inhibitors LAQ824 and LBH589 do not require death receptor signaling or a functional apoptosome to mediate tumor cell death or therapeutic efficacy. Blood. 2009;114:380-393. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Blagosklonny MV, Robey R, Sackett DL, Du L, Traganos F, Darzynkiewicz Z, Fojo T, Bates SE. Histone deacetylase inhibitors all induce p21 but differentially cause tubulin acetylation, mitotic arrest, and cytotoxicity. Mol Cancer Ther. 2002;1:937-941. [PubMed] [Cited in This Article: ] |
| 19. | Hirata H, Hinoda Y, Nakajima K, Kawamoto K, Kikuno N, Ueno K, Yamamura S, Zaman MS, Khatri G, Chen Y. Wnt antagonist DKK1 acts as a tumor suppressor gene that induces apoptosis and inhibits proliferation in human renal cell carcinoma. Int J Cancer. 2011;128:1793-1803. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Belinsky SA, Grimes MJ, Picchi MA, Mitchell HD, Stidley CA, Tesfaigzi Y, Channell MM, Liu Y, Casero RA, Baylin SB. Combination therapy with vidaza and entinostat suppresses tumor growth and reprograms the epigenome in an orthotopic lung cancer model. Cancer Res. 2011;71:454-462. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802:396-405. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Xu Q, Lu R, Zhu ZF, Lv JQ, Wang LJ, Zhang W, Hu JW, Meng J, Lin G, Yao Z. Effects of tyroservatide on histone acetylation in lung carcinoma cells. Int J Cancer. 2011;128:460-472. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Chuang C, Lin SH, Huang F, Pan J, Josic D, Yu-Lee LY. Acetylation of RNA processing proteins and cell cycle proteins in mitosis. J Proteome Res. 2010;9:4554-4564. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Wands JR. Prevention of hepatocellular carcinoma. N Engl J Med. 2004;351:1567-1570. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Zhuang H. Chronic hepatitis B virus infection, its treatment and prevention. Zhonghua Ganzangbing Zazhi. 2005;13:324-325. [PubMed] [Cited in This Article: ] |
| 26. | Tian L, He S, Li X, Hu WY, Peng PL, Wang F, Gao CY, Ren H, Tang KF. Long-fragment RNA inhibits hepatitis B virus gene replication and expression in HepG2.2.15 cells. Zhonghua Ganzangbing Zazhi. 2011;19:44-47. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Han YF, Zhao J, Ma LY, Yin JH, Chang WJ, Zhang HW, Cao GW. Factors predicting occurrence and prognosis of hepatitis-B-virus-related hepatocellular carcinoma. World J Gastroenterol. 2011;17:4258-4270. [PubMed] [DOI] [Cited in This Article: ] |